Physical History
Formation and Endurance of Life
By Mark Ciotola
First published on May 17, 2019. Last updated on January 19, 2021.
Recall the Contrasted Universe
Recall that due to expansion, the universe has become much cooler and darker over time. In fact, the typical temperature of the space between stars is nearly absolute zero. We have learned that heat energy tends to flow from warmer places to cooler places, as systems attempt to move towards thermal equilibrium (that is, until their temperatures are the same). Space is much cooler than stars, so energy tends to flow from within stars out into space. This is why we see stars shine.[1]
Planets are typically much cooler than stars. In fact, the temperature of a geologically dead, barren rocky planet would be about the same as space, that is nearly absolute zero. Shaded areas of moons and planets that lack atmospheres quickly drop to near zero. The side of the planet Mercury that faces away from the sun is such an example.
Solar System showing sun and planets (credit: NASA)
Yet planets orbiting around a star receive a significant continuing dose of energy in the form of light emitted from that star that then warms up the planet. The planet then becomes warmer than space, and so then the planet must start shedding energy into space. For example, the Earth receives significant amounts of sunlight that warms the earth. The Earth must then shed some of the energy into to attempt to move towards thermal equilibrium with space.[2]
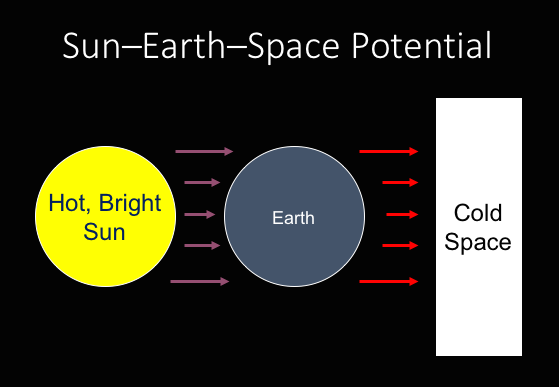
Sun-Earth-Space Potential
Heat Engine Analogy to Life
Recall the heat engine example. A heat engine bridges a temperature difference. Heat flows across that difference through the heat engine. Some of that heat energy is converted to work while the rest is exhausted as waste heat. Entropy is produced while the engine continues to function.
Part of the work done by a heat engine can be used to maintain that heat engine. More significantly, part of the work can go to build additional heat engines. These additional heat engines can produce yet more work to produce even more heat engines. The growth of heat engines is then exponential, at least until limiting factors come into play. This is a key point. Because heat engines can beget heat engines, an exponential increase in entropy production can take place.
Here, entropy production is proportional to the quantity of heat engines. Fast entropy favors exponential growth in entropy production, so fast entropy favors the “spontaneous” appearance and endurance of heat engines. Under the Second Law along, the spontaneous appearance of a heat engine is improbable but possible.[3]Fast entropy then utilizes those improbable appearances to create probable, self-sustaining, exponentially growing systems. Some of those systems have developed into what we call life.
Formation of Life
Steps
The motion of atoms and small molecules in a liquid or gas is nearly random. The statistics of these particles is known as statistical mechanics, or more traditionally, thermodynamics. The formation of life from this random motion involves several steps.
- Microscopic structures frequently appear by random chance. For example, atoms can combine to form molecules, and some molecules combine form to larger molecules.
- Even more complex microscopic structures occasionally appear by random chance.
- Some very complex microscopic structures form. Some of those forms will be durable.
- Some of those durable structures will be self-replicating. (Or they will be replicated by environment such as by catalysts). Such structures can be defined as the simplest form of life.
- Durable, self-replicating structures that degrade energy more quickly than their environment will be more probable (they will be favored under the principle fast entropy). Free energy will tend to be degraded through these structures.
- Where frequent chemical reactions can take place, where they can be durable and where there is an available source of free energy (such as from a thermodynamic potential), then the existence of the most basic life forms (as defined above) will approach being a certainty, given the passage of sufficient time.
Life Appears!

Jungle plants (credit: NASA)
Once these steps have occurred, life has developed. One can view life as the residue of random action subjected to the principle of fast entropy.
Summary
The Earth’s surface reflects some sunlight into space. Reflected light results in little entropy.
However, plants absorb much of that sunlight that would otherwise maintain its high level energy by being reflected into space.
Plants store some of that energy in the form of biomass. Animals have evolved to consume biomass.
Life As A Faster Path
Life itself can be viewed as the process of heat engines begetting heat engines. Bacteria are an easy example.
Hence, life represents a mechanism to maximize the rate of entropy production. Therefore, life is not due to pure luck; rather, the formation and evolution of life is favored under the e th Law.
Intelligence allows life to produce entropy even faster; thus the formation of increasingly powerful brains and intelligence are favored.
Notes and References
[1]That humans should have developed eyes that are particularly sensitive to the peak wavelengths emitted from the star our planet orbits should not be surprising.
[2]As long as the sun shines upon the Earth, the Earth will not reach thermal equilibrium with space. This continuing energy flow between the sun and the Earth maintains a continuing potential.
[3]I. Prigogine has proposed that dissipative structures can appear that increase entropy production. In his terminology, living organisms can be viewed as dissipative structures. Astrobiologist J. Lunine has paraphrased Prigogine’ s finding as follows: “complicated systems that are held away from equilibrium and have access to sufficiently large amounts of free energy exhibit self-organizing, self-complexifying properties.” (J. Lunine, Astrobiology, A Multidisciplinary Approach. Peason Addision Wesley, 2005).
« Energy Flows in Ecology | COURSE | Statistical and Evolutionary Intelligence »