Energy and Thermodynamics
Heat Engine with Exhaustible Reservoirs
By Mark Ciotola
First published on May 16, 2019. Last updated on April 29, 2021.
A heat engine
Recall that heat engine utilizes a temperature difference (a thermodynamic potential) to perform work Here we see a heat engine working between a warmer reservoir and a cooler reservoir (below). Warmth is represented by redder shading (in your version is in color) and greater height. The redder and higher the warmer heat reservoir, the hotter is it. Conversely, coolness is represented by bluer shading and lower height. The bluer and lower the cooler heat reservoir, the colder it is. Our heat engine begins operating between a quite hot and a quite cold reservoir as shown here.
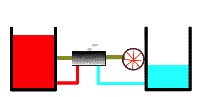
Heat engine operating upon “full” thermal reservoirs
As the heat engine continues to operate, the warmer heat reservoir becomes less hot and the cool reservoir becomes less cold (below).
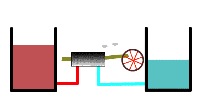
Thermal reservoirs partially depleted
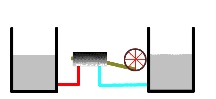
Thermal reservoirs completely depleted
Eventually, both the warmer and cooler heat reservoirs reach the same temperature (see figure). When this happens, no more work is possible. The heat engine is no longer operable. At this point, the reservoirs are said to be in thermal equilibrium.